 12-Sept-2018
12-Sept-2018
-
Organic Process Research &
Development publishes
online new findings opening the route to
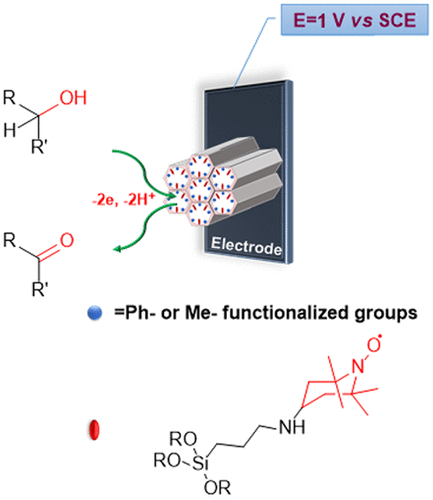
waste-free oxidation of alcohols into valued carbonyl compounds using
electricity only.
Developed in the context of Mina Ghahremani’s international doctorate
in organic
chemistry at the Institute for Advanced and Studies in Basic Sciences
(IASBS), Zanjan, Iran, under the joint supervision of Prof. Babak
Karimi and Dr Pagliaro in Italy, the new sol-gel electrode material
might be
used
to further green a
synthetic process (the catalytic oxidation of alcohols) that has seen
remarkable advances in the course of the last two decades.
The first sol-gel electrode based on organosilica functionalized with
the TEMPO moiety was
reported
in 2006 by the Italy's scholars, but it was only in 2015 that the first
practically useful electrode was
described in
pioneering work by Iran's scholars at IASBS. The latter electrode
enabled unprecedented turnover frequencies (TOF) of up to 3070 h
−1,
considerably higher than TOF reported until then for nitroxyl radicals
under chemical, electrochemical, or aerobic oxidation conditions. Alas,
the electrode showed poor catalytic stability.
The new
nanostructured electrode
now reported in OPRD, called by the joint team
"
ZEmOEl"
namely Zero-Emission
Oxidation Electrode, conjugates similar high selective activity with
unprecedented stability.
"
As remarked by Professor Kevin
Moeller", said in a comment
Professor Karimi, "
when electricity
to accomplish the oxidation originates from
photovoltaic modules or wind farms, as it is increasingly the case in
many regions of the world, these
electrodes
will enable to carry out a most important synthetic transformation with
solar energy only".
"
Enabling the use of solar energy to
multiple new processes" adds Dr Pagliaro, who is also a
distinguished scholar in solar energy, "
has been amongst our key
research objectives during our twenty years of research efforts in
Sicily".
The elegant electrochemical alcohol oxidation mediated by TEMPO-like
nitroxyl radicals has been reviewed by the international team in an
open access
study
published
in 2017 by
ChemistryOpen.
 12-Sept-2018
- Organic Process Research &
Development publishes
online new findings opening the route to
waste-free oxidation of alcohols into valued carbonyl compounds using
electricity only.
12-Sept-2018
- Organic Process Research &
Development publishes
online new findings opening the route to
waste-free oxidation of alcohols into valued carbonyl compounds using
electricity only.